Peptide-Based Covalent Inhibitors Bearing Mild Electrophiles to Target a Conserved His Residue of the Bacterial Sliding Clamp.
Compain, G., Monsarrat, C., Blagojevic, J., Brillet, K., Dumas, P., Hammann, P., Kuhn, L., Martiel, I., Engilberge, S., Olieric, V., Wolff, P., Burnouf, D.Y., Wagner, J., Guichard, G.(2024) JACS Au 4: 432-440
- PubMed: 38425897
- DOI: https://doi.org/10.1021/jacsau.3c00572
- Primary Citation of Related Structures:
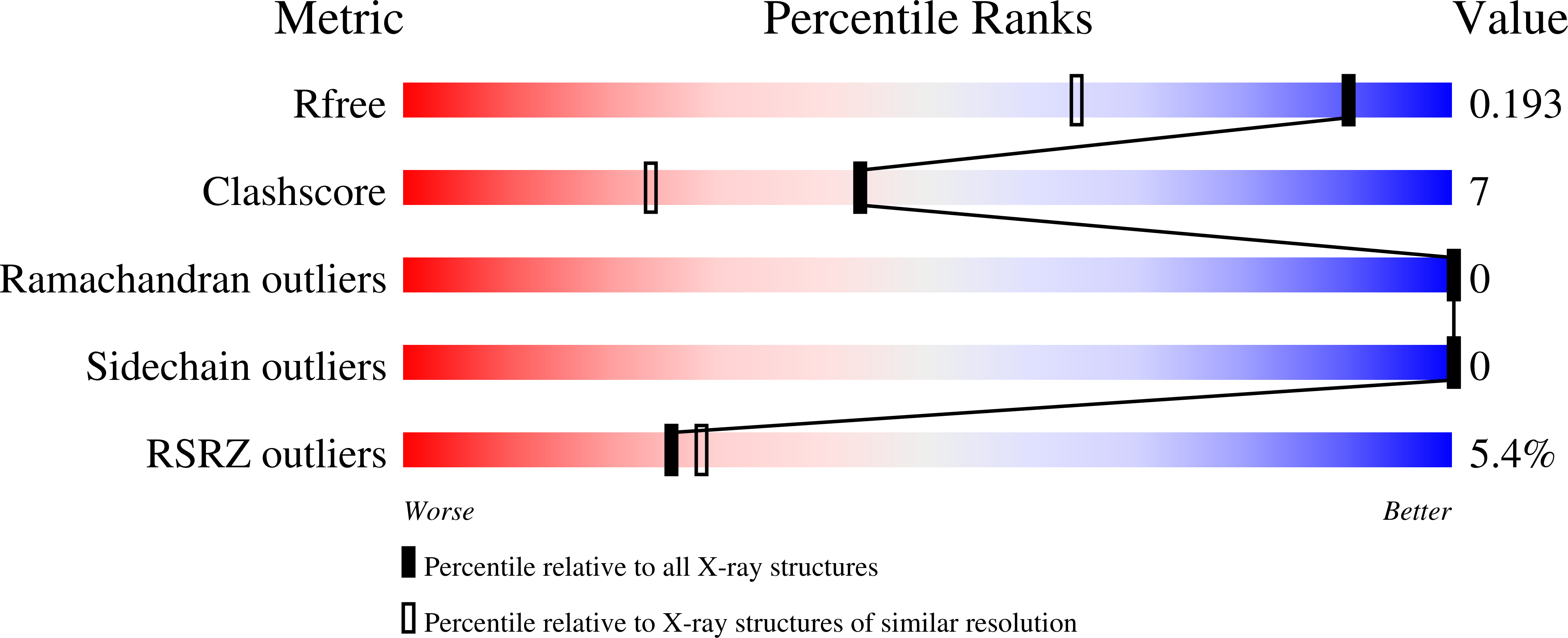
8PAT, 8PAY - PubMed Abstract:
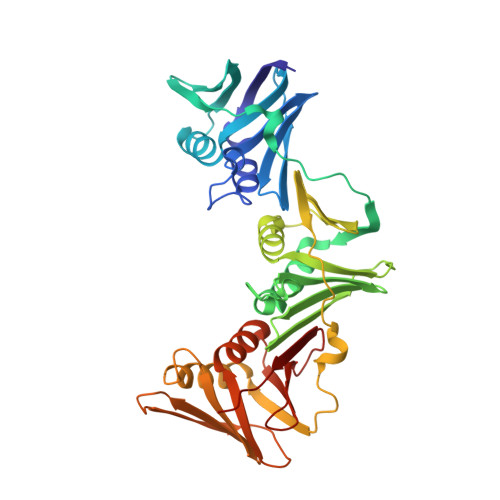
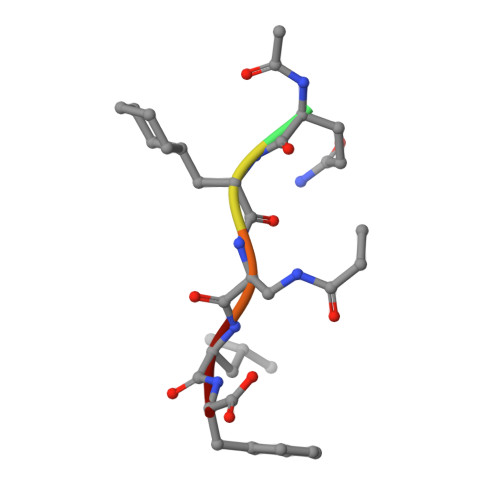
Peptide-based covalent inhibitors targeted to nucleophilic protein residues have recently emerged as new modalities to target protein-protein interactions (PPIs) as they may provide some benefits over more classic competitive inhibitors. Covalent inhibitors are generally targeted to cysteine, the most intrinsically reactive amino acid residue, and to lysine, which is more abundant at the surface of proteins but much less frequently to histidine. Herein, we report the structure-guided design of targeted covalent inhibitors (TCIs) able to bind covalently and selectively to the bacterial sliding clamp (SC), by reacting with a well-conserved histidine residue located on the edge of the peptide-binding pocket. SC is an essential component of the bacterial DNA replication machinery, identified as a promising target for the development of new antibacterial compounds. Thermodynamic and kinetic analyses of ligands bearing different mild electrophilic warheads confirmed the higher efficiency of the chloroacetamide compared to Michael acceptors. Two high-resolution X-ray structures of covalent inhibitor-SC adducts were obtained, revealing the canonical orientation of the ligand and details of covalent bond formation with histidine. Proteomic studies were consistent with a selective SC engagement by the chloroacetamide-based TCI. Finally, the TCI of SC was substantially more active than the parent noncovalent inhibitor in an in vitro SC-dependent DNA synthesis assay, validating the potential of the approach to design covalent inhibitors of protein-protein interactions targeted to histidine.
Organizational Affiliation:
Univ. Bordeaux, CNRS, Bordeaux INP, CBMN, UMR 5248, IECB, 2 Rue Robert Escarpit, F-33607 Pessac, France.