Identification and structural validation of purine nucleoside phosphorylase from Plasmodium falciparum as a target of MMV000848.
Chung, Z., Lin, J., Wirjanata, G., Dziekan, J.M., El Sahili, A., Preiser, P.R., Bozdech, Z., Lescar, J.(2023) J Biol Chem 300: 105586-105586
- PubMed: 38141766
- DOI: https://doi.org/10.1016/j.jbc.2023.105586
- Primary Citation of Related Structures:
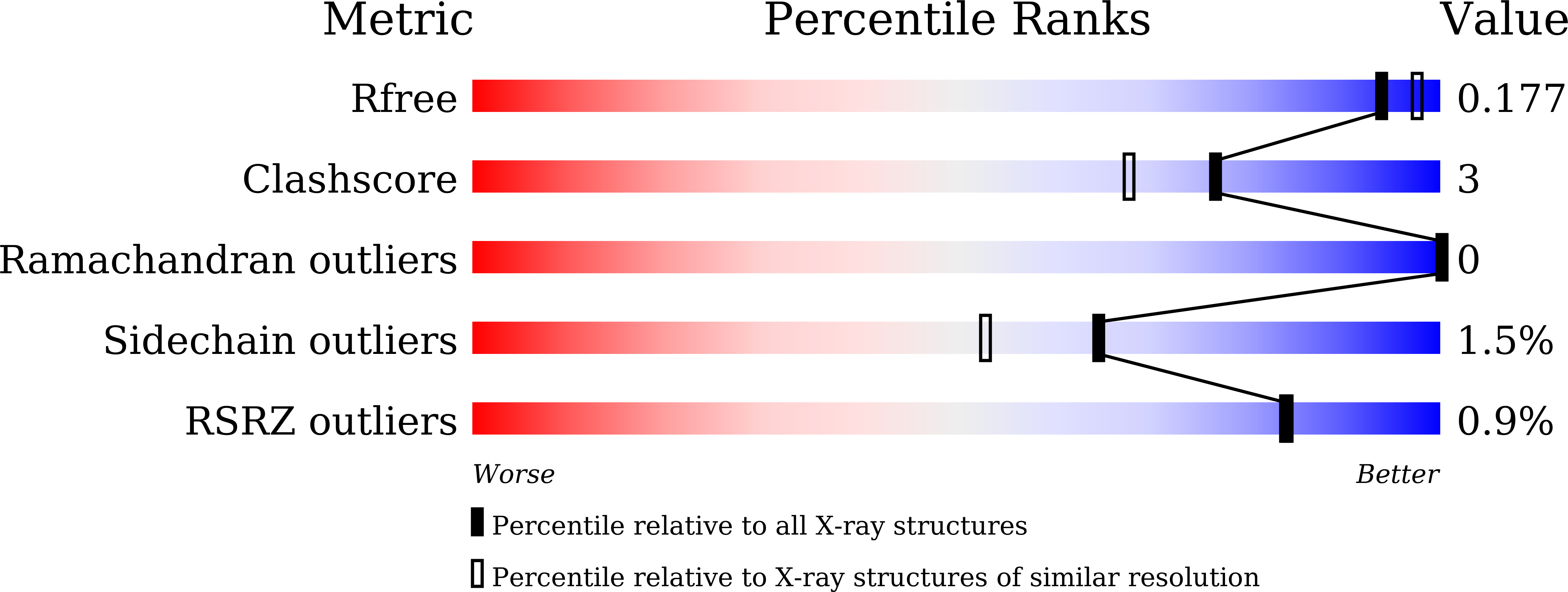
8W7H - PubMed Abstract:
About 247 million cases of malaria occurred in 2021 with Plasmodium falciparum accounting for the majority of 619,000 deaths. In the absence of a widely available vaccine, chemotherapy remains crucial to prevent, treat, and contain the disease. The efficacy of several drugs currently used in the clinic is likely to suffer from the emergence of resistant parasites. A global effort to identify lead compounds led to several initiatives such as the Medicine for Malaria Ventures (MMV), a repository of compounds showing promising efficacy in killing the parasite in cell-based assays. Here, we used mass spectrometry coupled with cellular thermal shift assay to identify putative protein targets of MMV000848, a compound with an in vitro EC50 of 0.5 μM against the parasite. Thermal shift assays showed a strong increase of P. falciparum purine nucleoside phosphorylase (PfPNP) melting temperature by up to 15 °C upon incubation with MMV000848. Binding and enzymatic assays returned a K D of 1.52 ± 0.495 μM and an IC 50 value of 21.5 ± 2.36 μM. The inhibition is competitive with respect to the substrate, as confirmed by a cocrystal structure of PfPNP bound with MMV000848 at the active site, determined at 1.85 Å resolution. In contrast to transition states inhibitors, MMV000848 specifically inhibits the parasite enzyme but not the human ortholog. An isobologram analysis shows subadditivity with immucillin H and with quinine respectively, suggesting overlapping modes of action between these compounds. These results point to PfPNP as a promising antimalarial target and suggest avenues to improve inhibitor potency.
Organizational Affiliation:
School of Biological Sciences, Nanyang Technological University, Singapore, Singapore; NTU Institute of Structural Biology, Experimental Medicine Building (EMB), Nanyang Technological University, Singapore, Singapore.