Crystal structure of rat haem oxygenase-1 in complex with ferrous verdohaem: presence of a hydrogen-bond network on the distal side
Sato, H., Sugishima, M., Sakamoto, H., Higashimoto, Y., Shimokawa, C., Fukuyama, K., Palmer, G., Noguchi, M.(2009) Biochem J 419: 339-345
- PubMed: 19154182
- DOI: https://doi.org/10.1042/BJ20082279
- Primary Citation of Related Structures:
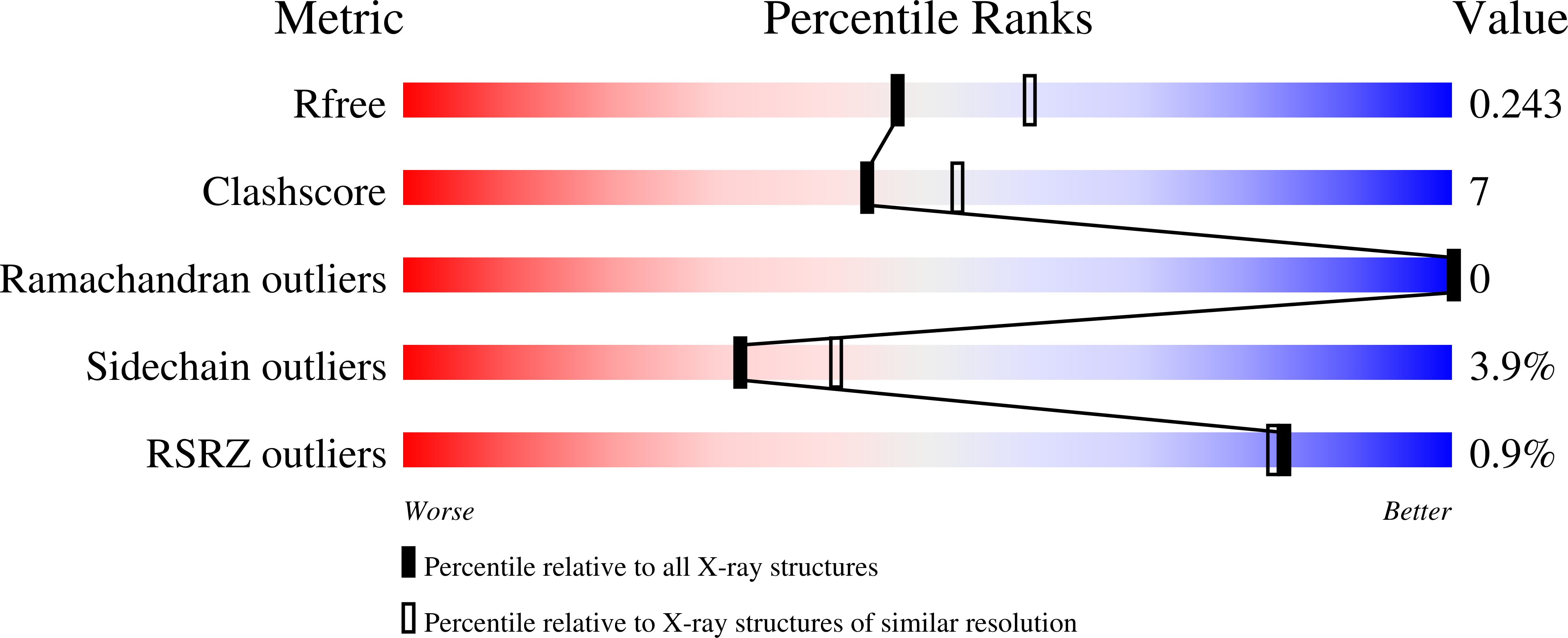
2ZVU - PubMed Abstract:
HO (haem oxygenase) catalyses the degradation of haem to biliverdin, CO and ferrous iron via three successive oxygenation reactions, i.e. haem to alpha-hydroxyhaem, alpha-hydroxyhaem to alpha-verdohaem and alpha-verdohaem to ferric biliverdin-iron chelate. In the present study, we determined the crystal structure of ferrous alpha-verdohaem-rat HO-1 complex at 2.2 A (1 A=0.1 nm) resolution. The overall structure of the verdohaem complex was similar to that of the haem complex. Water or OH- was co-ordinated to the verdohaem iron as a distal ligand. A hydrogen-bond network consisting of water molecules and several amino acid residues was observed at the distal side of verdohaem. Such a hydrogen-bond network was conserved in the structures of rat HO-1 complexes with haem and with the ferric biliverdin-iron chelate. This hydrogen-bond network may act as a proton donor to form an activated oxygen intermediate, probably a ferric hydroperoxide species, in the degradation of alpha-verdohaem to ferric biliverdin-iron chelate similar to that seen in the first oxygenation step.
Organizational Affiliation:
Department of Medical Biochemistry, Kurume University School of Medicine, 67 Asahi-machi, Kurume 830-0011, Japan.