Bioretrosynthetic construction of a didanosine biosynthetic pathway.
Birmingham, W.R., Starbird, C.A., Panosian, T.D., Nannemann, D.P., Iverson, T.M., Bachmann, B.O.(2014) Nat Chem Biol 10: 392-399
- PubMed: 24657930
- DOI: https://doi.org/10.1038/nchembio.1494
- Primary Citation of Related Structures:
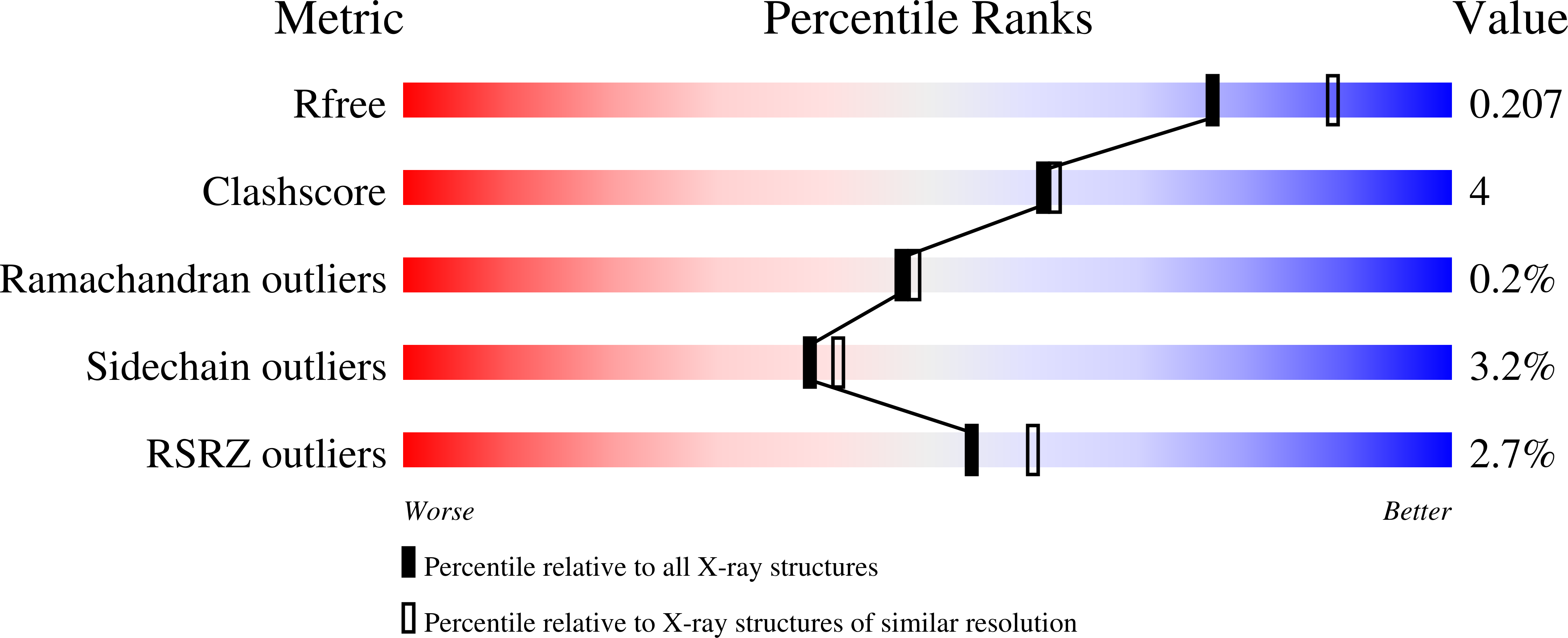
4LR7, 4LR8, 4LR9, 4LRA, 4LRB, 4LRC, 4LRD, 4LRE, 4LRF - PubMed Abstract:
Concatenation of engineered biocatalysts into multistep pathways markedly increases their utility, but the development of generalizable assembly methods remains a major challenge. Herein we evaluate 'bioretrosynthesis', which is an application of the retrograde evolution hypothesis, for biosynthetic pathway construction. To test bioretrosynthesis, we engineered a pathway for synthesis of the antiretroviral nucleoside analog didanosine (2',3'-dideoxyinosine). Applying both directed evolution- and structure-based approaches, we began pathway construction with a retro-extension from an engineered purine nucleoside phosphorylase and evolved 1,5-phosphopentomutase to accept the substrate 2,3-dideoxyribose 5-phosphate with a 700-fold change in substrate selectivity and threefold increased turnover in cell lysate. A subsequent retrograde pathway extension, via ribokinase engineering, resulted in a didanosine pathway with a 9,500-fold change in nucleoside production selectivity and 50-fold increase in didanosine production. Unexpectedly, the result of this bioretrosynthetic step was not a retro-extension from phosphopentomutase but rather the discovery of a fortuitous pathway-shortening bypass via the engineered ribokinase.
Organizational Affiliation:
1] Department of Biochemistry, Vanderbilt University Medical Center, Nashville, Tennessee, USA. [2].