Irregularities in enzyme assays: The case of macrophage migration inhibitory factor.
Cisneros, J.A., Robertson, M.J., Valhondo, M., Jorgensen, W.L.(2016) Bioorg Med Chem Lett 26: 2764-2767
- PubMed: 27156768
- DOI: https://doi.org/10.1016/j.bmcl.2016.04.074
- Primary Citation of Related Structures:
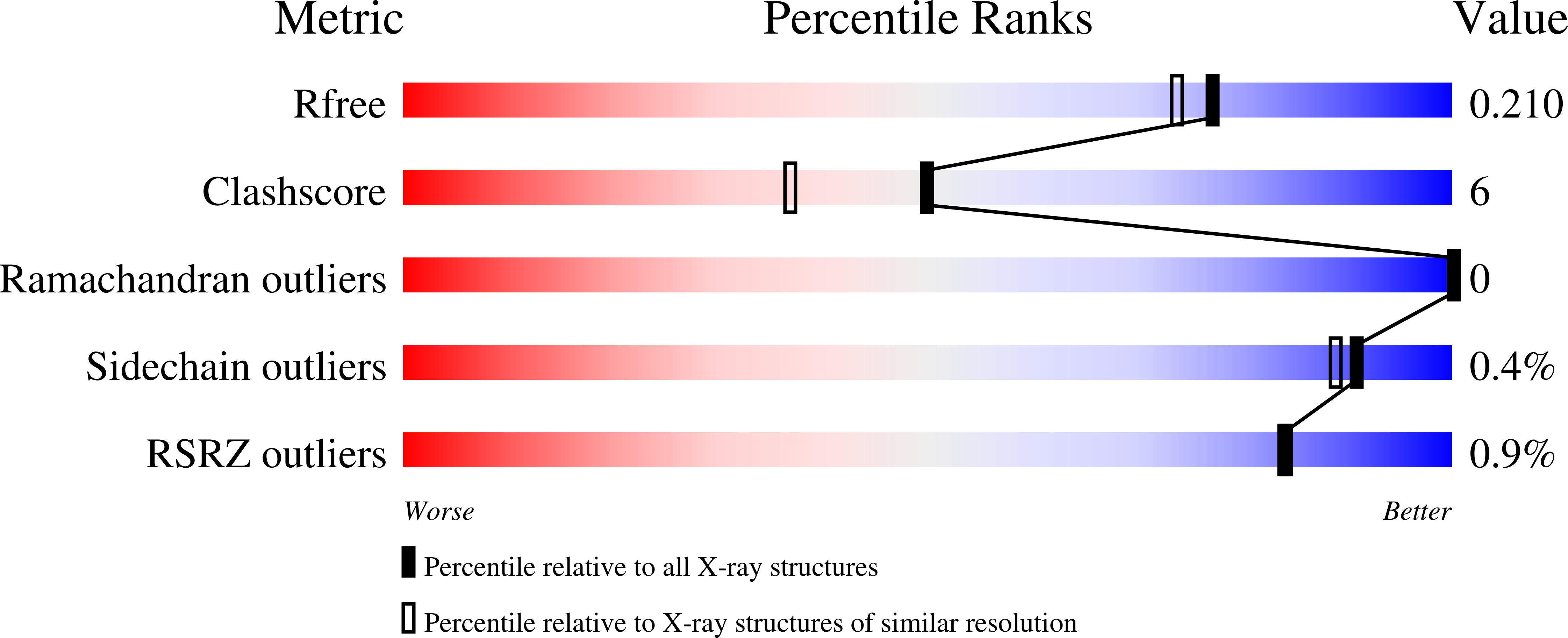
5J7P, 5J7Q - PubMed Abstract:
Inhibitors of human macrophage migration inhibitory factor (MIF) previously reported in the literature have been reexamined by synthesis, assaying for tautomerase activity, and protein crystallography. Substantial inconsistencies between prior and current assay results are noted. They appear to arise from difficulties with the tautomerase substrates, solubility issues, and especially covalent inhibition. Incubation time variation shows that 3, 4, 6, and 9 are covalent or slow-binding inhibitors. Two protein crystal structures are provided; one confirms that the twice-discovered 3 is a covalent inhibitor.
Organizational Affiliation:
Department of Chemistry, Yale University, New Haven, CT 06520-8107, United States.