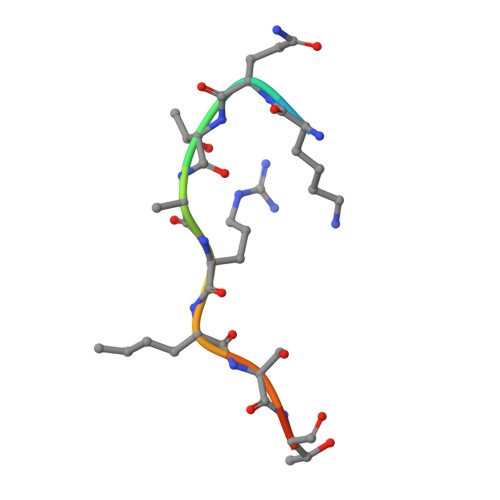
S-adenosyl methionine is necessary for inhibition of the methyltransferase G9a by the lysine 9 to methionine mutation on histone H3.
Jayaram, H., Hoelper, D., Jain, S.U., Cantone, N., Lundgren, S.M., Poy, F., Allis, C.D., Cummings, R., Bellon, S., Lewis, P.W.(2016) Proc Natl Acad Sci U S A 113: 6182-6187
- PubMed: 27185940
- DOI: https://doi.org/10.1073/pnas.1605523113
- Primary Citation of Related Structures:
5JHN, 5JIN, 5JIY, 5JJ0 - PubMed Abstract:
Lysine to methionine (K-to-M) mutations in genes encoding histone H3 are thought to drive a subset of pediatric brain and bone cancers. These high-frequency K-to-M mutations occur at sites of methylation on histone H3, and tumors containing the mutant histones exhibit a global loss of specific histone methylation marks. Previous studies showed that K-to-M mutant histones, also known as oncohistones, are potent orthosteric inhibitors of specific Su(var)3-9, Enhancer-of-zeste, Trithorax (SET) domain methyltransferases. However, the biochemical and biophysical details of the interaction between K-to-M mutant histones and the respective SET domain methyltransferases are currently unknown. Here, we use the histone H3K9-directed methyltransferase G9a as a model to explore the mechanism of inhibition by K-to-M oncohistones. X-ray cocrystal structures revealed that the K9M residue of histone H3 occupies the active site cavity of G9a, and kinetic analysis indicates competitive inhibition of G9a by histone H3K9M. Additionally, we find that the cofactor S-adenosyl methionine (SAM) is necessary for stable interaction between G9a and H3K9M histone. Consistent with the formation of a ternary complex, we find that the inhibitory peptide is uncompetitive with regard to SAM. These data and others indicate that K-to-M oncohistones promote global loss of specific lysine methylation through sequestration and inhibition of SAM-bound SET domain methyltransferases.
Organizational Affiliation:
Constellation Pharmaceuticals, Cambridge, MA 02142;