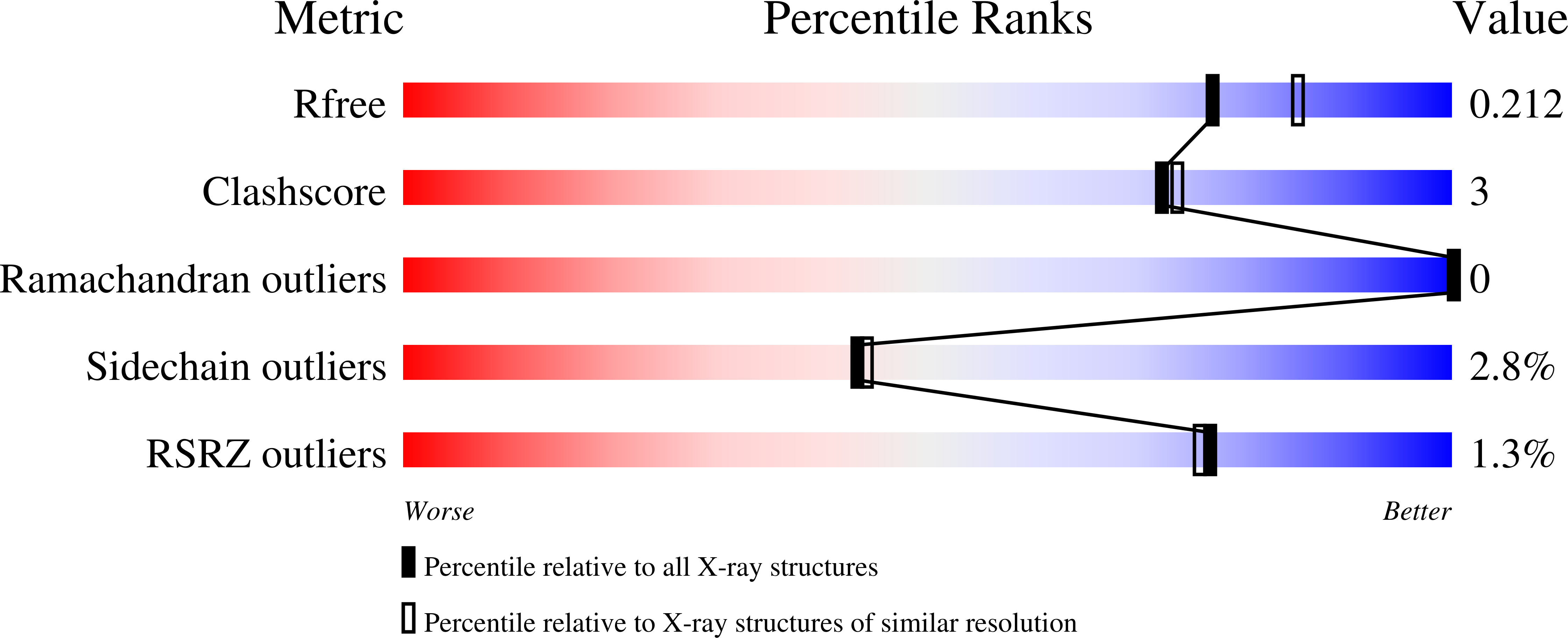
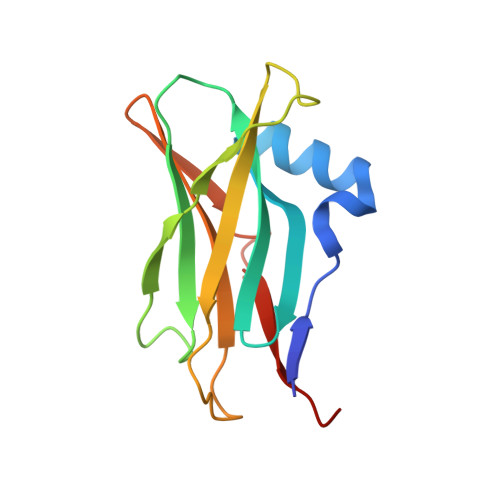
Molecular architecture of the glycogen- committed PP1/PTG holoenzyme.
Semrau, M.S., Giachin, G., Covaceuszach, S., Cassetta, A., Demitri, N., Storici, P., Lolli, G.(2022) Nat Commun 13: 6199-6199
- PubMed: 36261419
- DOI: https://doi.org/10.1038/s41467-022-33693-z
- Primary Citation of Related Structures:
7QF7, 7QFA, 7QFB, 7QM2 - PubMed Abstract:
The delicate alternation between glycogen synthesis and degradation is governed by the interplay between key regulatory enzymes altering the activity of glycogen synthase and phosphorylase. Among these, the PP1 phosphatase promotes glycogenesis while inhibiting glycogenolysis. PP1 is, however, a master regulator of a variety of cellular processes, being conveniently directed to each of them by scaffolding subunits. PTG, Protein Targeting to Glycogen, addresses PP1 action to glycogen granules. In Lafora disease, the most aggressive pediatric epilepsy, genetic alterations leading to PTG accumulation cause the deposition of insoluble polyglucosans in neurons. Here, we report the crystallographic structure of the ternary complex PP1/PTG/carbohydrate. We further refine the mechanism of the PTG-mediated PP1 recruitment to glycogen by identifying i) an unusual combination of recruitment sites, ii) their contributions to the overall binding affinity, and iii) the conformational heterogeneity of this complex by in solution SAXS analyses.
Organizational Affiliation:
Department of Cellular, Computational and Integrative Biology - CIBio, University of Trento, via Sommarive 9, 38123, Povo, Trento, Italy.